Need precise control over gene expression? Consider Clontech’s doxycycline-inducible systems. These systems offer tightly regulated gene switching, allowing you to study gene function with remarkable accuracy. We’ll explore their advantages and applications.
Doxycycline, a readily available antibiotic, acts as the key to unlock or silence gene expression in your cells. Clontech provides a range of vectors and cell lines optimized for this technology, providing flexibility for your research design. Choose from lentiviral, retroviral, or transfection-based systems depending on your experimental needs and cell type.
Key benefits include high inducibility, low background expression in the off-state, and broad compatibility across diverse cell lines. This results in cleaner data and more reliable interpretations of your experiments. Specific system selection hinges on your chosen cell line and the desired level of control. Consult Clontech’s detailed manuals for optimal protocol execution.
Troubleshooting tips: Ensure proper doxycycline concentration and incubation times; optimize cell density for your specific system; consider potential off-target effects. Remember, accurate titration of doxycycline is crucial to achieving the desired level of gene expression.
For detailed protocols, troubleshooting guidance, and a comprehensive list of compatible cell lines, refer to Clontech’s official website. Their resources offer valuable assistance and support.
- Clontech Doxycycline: A Detailed Guide
- Understanding Doxycycline-Inducible Systems from Clontech
- Choosing the Right System
- Optimizing Doxycycline Concentration and Treatment Duration
- Optimizing Doxycycline Induction for Efficient Gene Expression
- Applications and Case Studies of Clontech Doxycycline Systems
- Cancer Research
- Stem Cell Biology
- Drug Discovery and Development
- Further Considerations
Clontech Doxycycline: A Detailed Guide
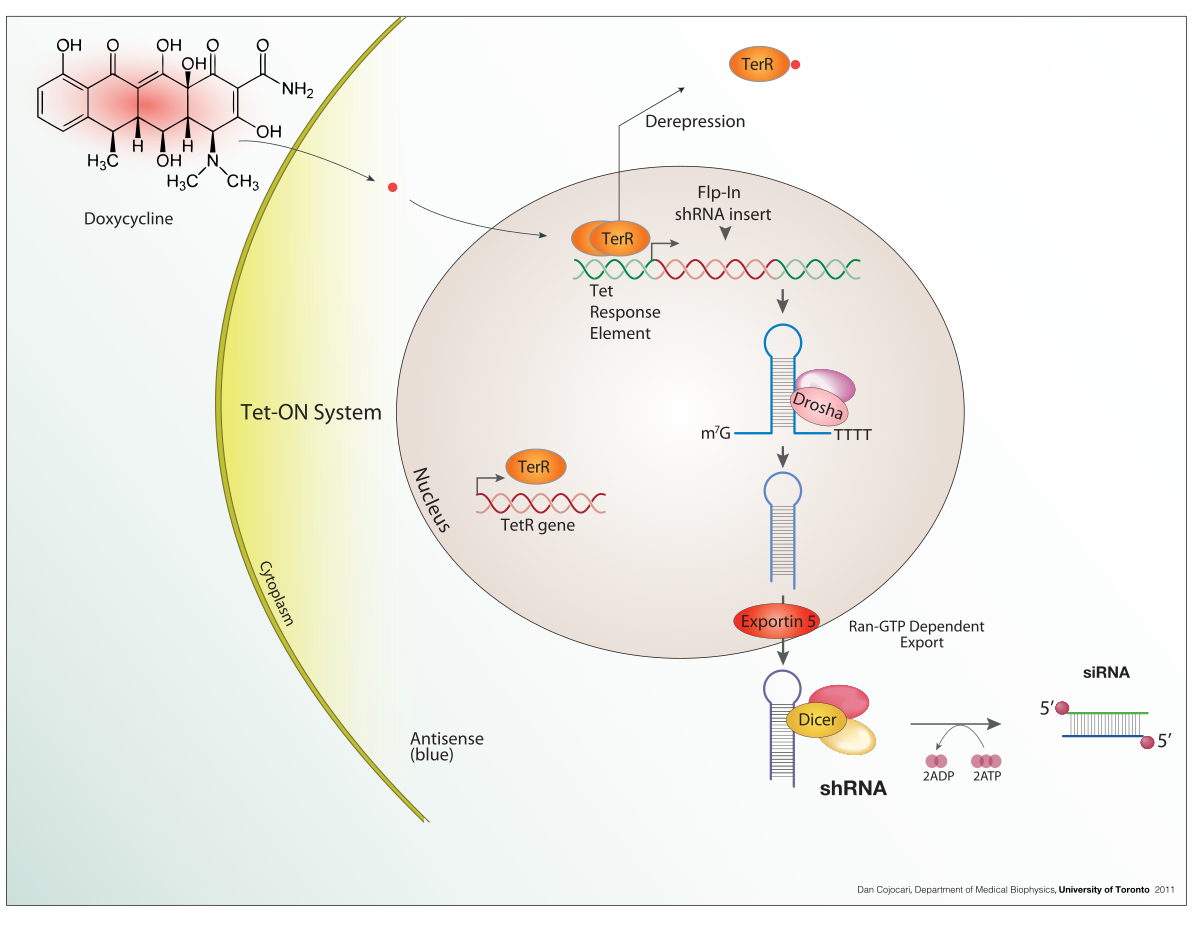
Use Clontech’s doxycycline according to the specific instructions provided with your chosen system. Concentrations vary depending on the application; refer to the product manual for optimal results. For Tet-On systems, doxycycline *induces* gene expression; for Tet-Off systems, it *represses* it.
Accurate dosing is paramount. Use a calibrated scale or micropipette to ensure precise measurements. Inconsistent dosing can lead to unreliable experimental results.
Proper storage is vital. Store doxycycline solutions according to the manufacturer’s recommendations, usually at -20°C for long-term storage and at 4°C for short-term use. Protect from light.
Monitor your cells regularly during induction or repression. Observe growth rates and morphology. Use appropriate controls (cells without doxycycline) to confirm the effects of the antibiotic.
Troubleshooting: If you observe unexpected results, review your doxycycline concentration, verify its storage conditions, and consider cell health. Contamination may also be a factor; ensure your cell culture practices are sterile.
Consider using a doxycycline-free control group to confirm the specificity of your results. This strengthens your conclusions and rules out non-specific effects.
Experiment with different doxycycline concentrations to determine the optimal range for your specific cell line and gene of interest. This optimization process is crucial for reproducibility.
Always document your methodology meticulously. Record the doxycycline concentration, incubation time, cell line, and any other relevant details. This information facilitates data interpretation and reproducibility.
For advanced applications like inducible shRNA systems, consult Clontech’s technical support or relevant literature. Specific protocols may differ from general guidelines.
Understanding Doxycycline-Inducible Systems from Clontech
Clontech offers several doxycycline-inducible systems, primarily relying on the Tet-On and Tet-Off systems. These systems utilize a tetracycline-controlled transactivator (tTA or rtTA) protein that binds to a tetracycline response element (TRE) promoter sequence, controlling gene expression. The Tet-On system activates gene expression in the presence of doxycycline, while the Tet-Off system represses gene expression in its presence. Choose the system best suited to your experimental needs based on whether you need inducible gene activation or repression.
Choosing the Right System
The choice between Tet-On and Tet-Off hinges on your specific experimental design. Tet-On is ideal for applications requiring tightly regulated gene induction, offering precise control over the timing and level of expression. Conversely, Tet-Off provides controlled gene repression, useful when studying gene functions requiring inhibition. Consider the level of leakiness acceptable for your application; some residual expression may be observed in the absence (Tet-On) or presence (Tet-Off) of doxycycline, depending on the specific system and cell type.
Optimizing Doxycycline Concentration and Treatment Duration
Doxycycline concentration is critical. Start with Clontech’s recommended concentration range and perform dose-response experiments to determine the optimal concentration for your specific cell line and gene of interest. Experiment with different treatment durations to find the optimal time point for desired gene expression levels. Prolonged doxycycline exposure may influence cell viability or gene expression in unforeseen ways, so careful titration is key.
Remember to carefully follow Clontech’s detailed protocols and troubleshooting guides provided with each system. Pay close attention to cell culture conditions and transfection methods for optimal results. Clontech offers technical support to assist with any issues you encounter during your experiments. Their resources will guide you through the process successfully.
Optimizing Doxycycline Induction for Efficient Gene Expression
Begin by titrating doxycycline concentration. Test a range (e.g., 0.1 µg/mL to 10 µg/mL) to determine the optimal concentration for your specific cell line and gene. This will yield the best expression level with minimal toxicity.
Next, consider the duration of doxycycline treatment. Experiment with different induction times (e.g., 24, 48, 72 hours) to identify the timeframe that maximizes gene expression without compromising cell viability. Careful monitoring of cell growth is key here.
Optimize cell density. Overcrowding can hinder gene expression. Aim for a confluency of 60-80% before adding doxycycline. Maintain appropriate cell culture conditions to support robust growth during induction.
Monitor gene expression using quantitative PCR (qPCR) or western blotting to confirm successful induction and measure expression levels at different doxycycline concentrations and induction times. This provides objective data to support your optimization strategy.
Finally, carefully select your Clontech Tet-On or Tet-Off system. The choice depends on your experimental design. Each system has specific parameters for optimal doxycycline induction, detailed in the respective system manuals. Adhere to the recommended protocols.
Applications and Case Studies of Clontech Doxycycline Systems
Clontech’s doxycycline-inducible systems offer precise control over gene expression, proving invaluable across diverse research areas. Let’s explore key applications and supporting studies.
Cancer Research
Researchers frequently use these systems to study oncogene function and tumorigenesis. For instance, a study in Oncogene (2018, Vol. 37, Issue 23) employed the Tet-On system to conditionally express MYC in mouse models, revealing critical insights into its role in lymphoma development. Specific experimental designs varied, highlighting the system’s adaptability.
- Investigate the impact of oncogene overexpression on cell growth and proliferation.
- Model tumor progression by inducibly activating oncogenes at specific developmental stages.
- Assess the effectiveness of novel cancer therapies by manipulating oncogene expression.
Stem Cell Biology
Precise control of gene expression is crucial in stem cell research. The Tet-Off system allows for tightly regulated differentiation by switching off crucial genes at specific time points. A publication in Cell Stem Cell (2015, Vol 17, Issue 2) demonstrated this by using the system to control expression of a lineage-specific transcription factor during hematopoietic stem cell differentiation. The results provided more precise control compared to other methods.
- Direct stem cell differentiation down specific lineages.
- Study the effects of gene overexpression or knockdown on stem cell self-renewal and differentiation.
- Generate isogenic cell lines with controlled variations in gene expression to study phenotypic differences.
Drug Discovery and Development
Clontech systems facilitate the study of drug targets and mechanisms of action. For example, inducible expression of a drug target allows for the study of its functional impact in a controlled manner, aiding in the evaluation of potential drug candidates. A report in Nature Biotechnology (2020, Vol. 38, Issue 10) showed this approach, using the system to modulate the expression of a specific kinase and evaluate the effects of various inhibitor compounds.
- Identify novel drug targets by studying the phenotypic consequences of gene manipulation.
- Evaluate the efficacy and specificity of drug candidates by manipulating their target’s expression.
- Study drug resistance mechanisms by inducing expression of resistance-conferring genes.
Further Considerations
Careful consideration of the appropriate system (Tet-On or Tet-Off) and cell type is essential for optimal results. Optimization of doxycycline concentration and induction time may be required depending on the specific experimental setup. Each system offers unique advantages and limitations dependent on research questions and model systems.